| T i p s |
| A bulletin in support of mushroom grower´s |
| May 2004 |
| DEVELOPMENT OF NON-COMPOSTED SUBSTRATES FOR PRODUCTION OF AGARICUS BISPORUS |
| J. Ernesto Sánchez Vázquez (1), Daniel. J. Royse (2) and G. Hernández (1) |
| Mushroom Biology and Mushroom Products. Sánchez et al. (eds). 2002 UAEM. ISBN 968-878-105-3 |
| ABSTRACT A pasteurized mixture of non-composted raw materials was formulated to produce a brown variety of the common cultivated mushroom Agaricus bisporus. Yield and % biological efficiency ranged from 17.2 kg/m2 (42%) to 31.4 kg/m2 (77%), depending on experimental conditions. The addition of non-composted sheep manure to the substrate increased yield. This procedure enables mushroom growers to produce portabello mushrooms on pasteurized non-composted substrates. |
| 1 El Colegio de la Frontera Sur, P.O. Box 36. Tapachula, Chiapas 30700 México. <esanchez@tap-ecosur.edu.mx> 2 Department of Plant Pathology, The Pennsylvania State University 316 Buckhout Laboratory,, University Park, PA 16802 USA. <djr4@psu.edu> |
| INTRODUCTION Currently, all commercial cultivation of Agaricus bisporus is based on the production of selective compost prepared in a two-phase process. Phase I involves the aerobic breakdown of raw materials while phase II involves pasteurization and conditioning of the compost to provide for a selective growth medium (Sinden and Heuser 1953, Wuest 1977, Overtjins 1998). This process has been adopted worldwide because of its many advantages, including selectivity of the substrate for the mushroom, economically acceptable yields and quality of mushrooms at low cost, and because it is adapted to large-scale production. However, this two phase process has some important disadvantages: 1) it is time, space and labor consuming, 2) it requires high initial investment, and 3) it is not always environmentally-friendly. A technical altemative to the commonly used composting method was introduced in 1962, when Till reported that it was possible to produce A. bisporus on a non-composted, sterilized (121Celsius degrees, two hours) sawdust based mixture. Later, Murphy (1972) reported on his efforts to produce this mushroom without phase I composting and Mee (1978) demonstrated that it was also possible to produce A. bisporus on dried, non-composted "cold" manure. Finally, Sánchez and Royse (2001) reported a procedure for preparing mushroom substrate without composting on ingredients used for the cultivation of shiitake. Thus, it clearly is possible to cultivate A. bisporus on non-composted substrate; however more research is needed to improve formulas and techniques used for its production. Our work explores a wider range of substrates for cultivating this mushroom. |
| MATERIALS AND METHODS Strain Commercial brown strain CM-444 of A. bisporus from The Pennsylvania State University Mushroom Culture Collection was used for the production of portabello. Substrates for mycelial growth The linear extension rate (LER) of strain 444 was determined by conducting two sets of experiments. The first experiment consisted of different mixtures of peat/limestone supplementedwith various types of "cold" manure as shown in Table 1. The second experiment consisted of a mixture of different organic by-products and supplements as follows (% oven dry ingredients): corn cobs (4%), sorghum (22%), gypsum (2%), soybean (5%), wheat bran (10%), sheep manure (4%), sawdust (15%) peat moss (8%). Coffee pulp and grass were added to this mixture in the proportions shown in Table 2. Sterilization was completed in an autoclave at 121?C for 15 minutes. After cooling, three grains of sorghum spawn were placed in the bottom of sterile 16 x 180 mm tubes (at least 5/treatment) and 10 g of moistened sterile substrate were added. The tubes then were incubated at 25?C for 20 to 25 days. Mycelial growth was measured every two days. Substrate for fruiting Two mixtures were defined with ingredients as follows (% oven dry ingredients): 1) basic mixture (Sánchez and Royse 2001; BM, Table 3) containing oak sawdust (28%), millet (29%), rye grain (8%), peat moss (8%), alfalfa meal (4%), soybean flour (4%), wheat bran (9%), and CaC03 (10%) and 2) altemative mixture (AM, Table 4) containing corn cobs (4%), sorghum (22%), gypsum (2%), soybean (5%), wheat bran (10%), sheep manure (4%), sawdust (15%), coffee pulp (5%), grass (25%) and peat moss (8%). Ingredients were modified as shown in Table 4. The straw-bedded sheep manure was collected, semidried at room temperature and then further dried for 48 h at 80?C. Dried material then was ground (Wiley mill; 1 mm screen) to a fine powder before use. Coconut fíber ("Cocopeat") was kindly supplied by Agroindustrias La Moderna while sawdust was collected from a sawmill milling Cybitax donnel. Cultivation methods Standard cultivation methods for A. bisporus were used as outlined by Wuest and Bengston (1982), except that a non-composted substrate was used. The ingredients were mixed, moistened (55%), thermally treated (110?C, 20 min), cooled and spawned with 0.8-1% (wet wt) sorghum grain spawn. After spawning, the substrate was bagged in 1 kg sterile bags with a patch fílter (Unicorn Import and Manufacturing Co, Commerce, TX) and then heat-sealed. Temperature during spawn run was maintained for 2 to 3 wk at 18?C (experiments in Table 3) and at 25?C (experiments in Tables 4 and 5). Bags were opened and 2.5 cm of CAC'ed casing was over-laid on the substrate surface (Tschierpe 1990). Case hold lasted 2 to 3 weeks at 18 ?C for the 1st set of experiments (Table 3) and at 22-23?C for the 2nd and 3th set (Tables 4 and 5). During case hold, water was applied daily as outlined by Schisler and Wuest (1978). Parameters evaluated Mycelial growth measurements were plotted against time and the regression line for each treatment was calculated (Statistica, StatsSoft Inc, USA versión 5.5). The slope of each line was defined as the linear extension rate (LER). Total weight of mushrooms per bag (TWM), biological efficiency [BE= weight (kg) of fresh mushroom per kg of dry substrate] and the yield (kg/m) were determined after the 3ra break. Experimental design and statistical analysis For LER, a multivariate comparison of slopes was made (Kleinbaum et al. 1998) applying acorrection of the signifícance level by the Bonferroni procedure (Simes 1986). For determining the predictíon profile of mfluence of ingredients on growth, a Mixture Design using JMP 4.0 software from SAS (1996) was used. For fruiting parameters, a completely randomized design with ten replicas was used. The ANOVA and means separation was performed using the GLM procedure (SAS 1998). |
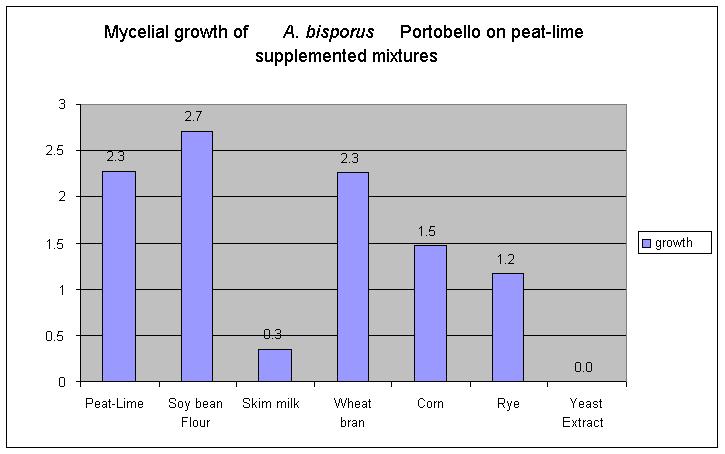
| RESULTS Mycelial growth The LER of CM-444 on three different types of "cold" manure is shown in Table 1. The highest LERs were attained when sheep manure was used in ratios of 1:1 and 1:0.5 with peat (4.28 and 3.77 mm/day, respectively). Cow manure/peat in a ratio 1:1 and the control-CSH+SWDST formed a second group (2.48 and 2.08 mm/day, respectively). The last group was formed by the two treatments containing pig manure, where no growth was observed. |
| Table 1. Linear extensión rate of Agaricus bisporus portabello CM-444 (brown strain grown at 25ºC) on peat supplemented with three types ofcold manure. Treatments Linear extensión rate ________________________________________(mm/day)*____ Sheep manure: peat 1:0.5 4.28 a Sheep manure:peat 1:1 3.77a Cow manure :peat 1:1 2.48 b Mixture (CSH+SWDST) 2.08 b Cow manure:peat 1:0.5 1.56 c Peat-lime (control) 1.11 c Pig manure:peat 1:0.5 0 d Pig manure:peat 1:1 0 d * Means followed by the same letter are not significantly different (a= 0.05 after Bonferroni bound). |
| The LER and colony diameter (14 da) obtained on different levéis ofcoffee pulp and grass is shown in Table 2. The highest LER was obtained in treatments that formed the fírst group (1, 3, 8, 6 and 9, with 2.82, 2.87, 2.72, 2.61 and 2.57 mm/day, respectively). Figure 1 shows the prediction profile for the growth of CM-444 when ingredients in the substrate in Table 2 (AM, coffee pulp and grass) are modified. The best growth was obtained when the ingredients were in the following proportions: AM = 85%, coffee pulp = 5% and grass = 10%. |
| Figure 1. Prediction profile for influence of various proportions of coffee pulp and grass mixtures on growth of Agaricus bisporus CM-444. Biological efficiency and yield Total weight, BE and yield of CM-444 when grown on the BM and compared to a mixture containing 4% dried straw-bedded sheep manure substituted for the same amount of sawdust is shown in Table 6. The average total weight of mushrooms obtained from each bag (2.65 kg) of substrate was 558.8 g for BM and 870.3 g for the treatment containing sheep manure. The biological efficiency was 42 and 66% and the estimated yield was 17.2 and 26.8 kg/m2, respectively. Table 3. Total weight of mushrooms (TWM), yield and biological efficiency (BE) of CM 444 portabello on non-composted substrate with and without sheep manure. TWM* BE* Yield (g) (%) (kg/m2) Basic mixture 558.8b 42.17 17.20 BM + Sheep manure 4% 870.3a 65.68 26.80 *Means followed by the same letter are not signifícantly different according to the Waller-Duncan k-ratio t test (P=0.05). CV=18.3%. Substrate wet wt= 2.65 kg Comparisons of total mushroom weight (TWM), yield and biological effíciency (BE) of CM-444 portabello grown on a non-composted mixture (AM) with and without com or rice grain is shown inTable 4. The average total weight of mushrooms obtained from each bag (1 kg wet wt) was 147.1, 167.7 and 177.7 kg for AM, AM supplemented with rice, and AM supplemented with corn, respectively. Máximum BE was obtained from AM supplemented with corn or rice (39.5% and 37.2% respectively). Mushroom yields varied between 13.7 kg/m2 and 12.9 kg/m2 (corn and rice respectively) to 11.3 kg/m2 on the non-supplemented mixture. Table 4. Total weight, yield and biological effíciency (%BE) of CM-444 portabello on a non-composted mixture (AM) with and without corn or rice grain. TWM* Yield Treatment (g) BE (%) (kg/m2) AM 147.1 b 32.7 11.3 AM+Rice grain 10% 167.7a 37.2 12.9 AM+ corn grain 10% 177.7a 39.5 13.7 *Means followed by the same letter are not significantly different according to the Waller-Duncan k-ratio t test (P=0.05). CV= 14.2%. Substrate wet wt = 1 kg |
| DISCUSSION Our results confirm the possibility of obtaining acceptable yields of A. bisporus on a non-composted substrate. Our yields were equivalent or better than those obtained in commercial cropping houses using Phase II compost. Average yields from Phase II compost facilities in the United States were approximately 16.8 ± 4 kg/m2 in 1987 (Rinker and Wuest). This work also increases the range of materials usable for the production of mushrooms as reported by Sánchez and Royse (2001). The use of non-composted sheep manure in the BM improved yield. This agrees with the fíndings of Mee (1978) who reported the growth of A. bisporus on a pasteurized mixture of peat and cold manure. Mee (1978) aiso suggested the use of steer, cow or pig manure. The reason(s) for lack of growth of A. bisporus on pig manure in our experiments is not known. It is possible that the pig manure contained some ammonia, although none was obvious at the time of spawning. Addition of coffee pulp (up to 5% of the total substrate wt) increased LER; however, further additions had a negative impact. This agrees with previous work done on other mushroom species (Calvo et al. 1995). He showed that 3% coffee pulp improved LER of Auricularia fuscosuccinea but higher rates inhibited growth. Morales et al. (2000) aiso found that primordium growth and development was affected in the same way. Variation of the grass content in substrate mixtures had no appreciable effect on LER; thus, grass may be used as one of the ingredients in substratos but offers no improvement in yields over other ingredients. Grass may be more readily available than other ingredients in some parts of the country. Most of these experiments were conducted at 23 Celsius degrees, which is higher than the optimal production temperature for the strain used. This may have reduced BEs that were generally lower than those obtained in our previous work (77.1%; Sánchez and Royse 2001). Additional work may lead to the identification of other basal ingredients and nutrient supplements that wouid further increase yields and BEs. |
| REFERENCES Block, S.S. and S.N. Rao. 1962. Sawdust compost for mushroom growing. Mush Sci 5:134-141. Calvo-Bado, L.A., J.E. Sánchez-Vázquez y G. Huerta-Palacios. 1995. Evaluación de diversos sustratos para el crecimiento micelial de Auricularia fuscosuccinea (Mont.) Farlow. Rev. Mic. Neotrop. Apl. 8: 27-37. Kleinbaum, D.G, L.L. Kupper, and K.E. Mueller. 1998. Applied regression analysis and multivariate methods. Ed. PWS-Kent Pub. Co. 276-283. Mee, H.M. 1978. US Patent 4 127 964. Morales, G.E., G. Huerta-Palacios and J.E. Sánchez-Vázquez. 2000. Production technology optimization for Auricularia fuscosuccinea . Mush. Sc. 15: 943- 948. Murphy, W.S. 1972. Development of a mushroom production medium without phase I composting. Mush. News20:(12) 4-22. Overtijns, A. 1998. The conventional Phase II in trays or shelves. Mush J. 584:15-21. Rinker, D.L. and P.J. Wuest. 1987. Cultural and environmental factors influencing commercial mushroom production in Pennsylvania. Dev Crop Sci. 10:637- 640. Royse, D.J. 1996. Yield stimulation of shiitake by millet supplementation of wood chip substrate. In: Royse, D.J. (Ed.) Proceedings of the Second International Conference on Mushroom Biology and Mushroom Products, June 9-12, University Park, PA. 277-283. Royse, D.J. 1997. Specialty mushrooms and their cultivation. Hort Rev. 19:59-97. Sánchez, J.E. and D J. Royse. 2001. Adapting substrate formulas used for shiitake for production of brown Agaricus bisporus. Bioresource Technol. 77:65- 69. SAS Institute. 1996. JMP Start Statistics. SAS Institute, Statistical Analysis System, Cary, NC. SAS Institute. 1998. SAS User's Guide: Statistics. SAS Institute Statistical Analysis System, Cary, NC. Schisler, L.C. and P.J. Wuest. 1978. Watering and ventilation from casing through cropping in commercial mushroom production. Special Circular 140. The Pennsylvania State University. University Park. Simes, R.J. 1986. An improved Bonferroni procedure for multiple tests of significance. Biometrika 73:751-754. Sinden, J.W. 1990. Developments in spawn production. Mush. News. 38 (10):6-11. Sinden, J.W. and E. Heuser. 1953. The nature of the short composting process and its relation to short composting. Mush Sci. 2:123-131. Sinden, J.W. and L.C. Schisler. 1962. Nutrient supplementation of mushroom compost at spawning. Mush. Sci. 5:267-280. Tschierpe, H.J. 1990. Cacing, the elegant method to influence crop rhythm. In: Proceedings Australian and New Zealand National Mushroom Industry Conference, Mushroom Growers Association, Australia. 96-104. Till, O. 1962. Champignonkultur auf sterilisiertem naehrsubstrat und die wiederverwendung von abgetragenem kompost. Mush Sci. 5:127-133. United States Department of Agriculture. 2001. Mushrooms. Agri. Stat. Bd, Washington. Wiegant, W.M., J. Wery, D. Buitenhuis and J.A.M. de Bont. 1992. Growth-promoting effect of thermophilic fungi on the mycelium ofthe edible mushroom Agaricus bisporus. Appl Environ Microbiol 65:2654-2659. Wuest, P.J. 1977. Compost and the composting technique. Mush. News 2.(5):11-16. Wuest, P.J. and G.D. Bengston. 1982. Penn State Handbook for Commercial Mushroom Growers. Special Publication, College of Agricultural Sciences, Pennsylvania State University. 129. |
| May 2004 |
 |
 |
| Spawn running in bags |
| . |
| Mushrooms ready for harvest |
| . |
 |
| ACKNOWLEDGEMENTS The authors would like to thank Tom Rhodes, Doug Keith and Henry Shawley of the Mushroom Research Center, P.S.U. and J. Valle from ECOSUR for their techmcal assistance in the conduct of this work. |
| Table 2. Linear extensión rate (LER) and colony diameter (14 days/25 Celsius degress) of Agaricus bisporus CM-444 grown on various mixtures containing coffee pulp and grass. |
| Substrate composition (%) Mean Extension on Treatment AM Cofee pulp grass LER* (mm/day) day 14 (mm) 1 75 5 20 2.82 a 32.67 2 100 0 0 2.04 c 24.17 3 70 10 20 2.87 a 29.25 4 95 5 0 2.13 c 24.33 5 90 10 0 2.08 c 23.42 6 90 0 10 2.61 ab 28.92 7 80 10 10 2.31 be 29.08 8 85 5 10 2.72 a 32.78 9 80 0 20 2.57 ab 30.67 Means followed by the same letter are not significantly different (a= 0.05) according to the Tukey test. CV= 15.3%. |